Introduction
The Robinson Annulation is a pivotal reaction in organic chemistry, especially in the synthesis of complex cyclic compounds. Named after Sir Robert Robinson, this reaction is a powerful tool for constructing six-membered rings, which are foundational in many natural products and pharmaceuticals. Understanding the starting materials and mechanism of this reaction is essential for both academic study and practical application in chemical synthesis.
This blog delves into the details of the Robinson Annulation, focusing on the two starting materials required for the reaction, their roles, and how they contribute to the formation of the final cyclic product. We’ll also explore the reaction mechanism, applications, and tips for optimizing this reaction.
Overview of the Robinson Annulation
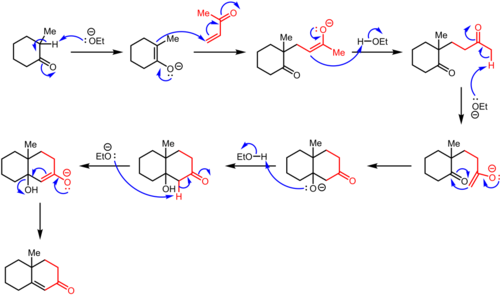
The Robinson Annulation is a two-step reaction that combines a Michael addition with an intramolecular aldol condensation, leading to the formation of a six-membered cyclic compound.
- Reaction Type: Carbon-carbon bond-forming reaction.
- Final Product: A cyclohexenone derivative.
- Applications: Widely used in the synthesis of steroids, alkaloids, and other complex natural products.
What Are the Two Starting Materials?
The Robinson Annulation requires the following two starting materials:
- Enolate or Enolate Equivalent
- Typically derived from a 1,3-dicarbonyl compound such as a β-keto ester or β-diketone.
- Example: Ethyl acetoacetate, acetylacetone.
- Role: Acts as a nucleophile during the Michael addition step.
- α,β-Unsaturated Carbonyl Compound
- Generally an enone or enal, such as methyl vinyl ketone or cinnamaldehyde.
- Example: Methyl vinyl ketone (CH₂=CH-COCH₃).
- Role: Serves as the Michael acceptor, facilitating the addition of the enolate nucleophile.
Reaction Mechanism of the Robinson Annulation
The Robinson Annulation proceeds through the following steps:
1. Michael Addition
- The enolate (or enolate equivalent) reacts with the α,β-unsaturated carbonyl compound via a Michael addition.
- This step forms a 1,5-dicarbonyl intermediate.
2. Intramolecular Aldol Condensation
- The 1,5-dicarbonyl intermediate undergoes base-catalyzed intramolecular aldol condensation.
- A new carbon-carbon bond is formed, creating a six-membered ring.
3. Dehydration
- The aldol product loses a molecule of water, resulting in the formation of a cyclohexenone derivative.
Key Factors Affecting the Reaction
- Choice of Base:
- Common bases include NaOH, KOH, or alkoxides like sodium ethoxide. The base facilitates enolate formation and promotes aldol condensation.
- Temperature:
- Lower temperatures are preferred for the Michael addition to avoid side reactions.
- Higher temperatures may be needed during the aldol condensation step.
- Solvent:
- Polar aprotic solvents, such as DMF or DMSO, are often used to dissolve polar intermediates and enhance reaction efficiency.
- Substrate Compatibility:
- Both the enolate and α,β-unsaturated carbonyl compound must be reactive and free from steric hindrance.
Applications of the Robinson Annulation
- Natural Product Synthesis:
- Used extensively in constructing the core structure of steroids, terpenes, and alkaloids.
- Pharmaceutical Chemistry:
- Enables the synthesis of key intermediates for drug molecules.
- Material Science:
- Cyclohexenone derivatives obtained from this reaction are used in designing functional materials.
- Synthetic Organic Chemistry:
- A fundamental tool in retrosynthetic analysis and the design of complex molecule syntheses.
Practical Example: Synthesis of a Cyclohexenone
Materials:
- Enolate: Ethyl acetoacetate.
- Michael Acceptor: Methyl vinyl ketone.
Procedure:
- Dissolve ethyl acetoacetate in ethanol with a catalytic amount of sodium ethoxide.
- Add methyl vinyl ketone dropwise while stirring.
- Maintain the reaction mixture at room temperature for the Michael addition.
- Heat gently to induce aldol condensation, followed by dehydration.
- Purify the product to obtain the cyclohexenone derivative.
Challenges and Troubleshooting
- Side Reactions:
- Over-alkylation can occur if an excess of the Michael acceptor is used.
- Incomplete Reaction:
- Ensure the base is fresh and active to facilitate enolate formation.
- Product Isolation:
- Use appropriate purification techniques, such as recrystallization or column chromatography, to isolate the desired product.
Advantages of the Robinson Annulation
- Efficiency:
- Combines two key reactions into a single process.
- Versatility:
- Compatible with a wide range of starting materials, allowing for diverse product formation.
- Scalability:
- Can be scaled up for industrial synthesis without significant modifications.
Alternatives to the Robinson Annulation
- Aldol Reaction Alone:
- For simpler cyclic ketone synthesis without the Michael addition step.
- Michael Addition with Separate Cyclization:
- Allows for more control over reaction intermediates.
- Diels-Alder Reaction:
- An alternative for synthesizing cyclic compounds but involves a different reaction mechanism.
Frequently Asked Questions (FAQs)
Q1: What makes the Robinson Annulation unique?
A: The reaction combines a Michael addition and aldol condensation into a single process, efficiently forming six-membered rings.
Q2: Can I use other enolates besides β-keto esters?
A: Yes, other enolates such as β-diketones and malonates can be used, depending on the desired product.
Q3: What are common challenges in performing this reaction?
A: Side reactions, incomplete cyclization, and difficulty in product isolation are common issues.
Q4: Is the Robinson Annulation environmentally friendly?
A: While the reaction itself is efficient, the use of organic solvents and bases requires careful disposal to minimize environmental impact.
Conclusion
The Robinson Annulation is a cornerstone reaction in organic chemistry, enabling the efficient synthesis of cyclohexenone derivatives. By understanding the roles of the two starting materials—an enolate and an α,β-unsaturated carbonyl compound—you can harness this reaction for diverse applications in natural product synthesis, pharmaceuticals, and beyond. With proper handling, optimization, and troubleshooting, the Robinson Annulation remains an indispensable tool for chemists worldwide.